Ultraviolet Radiation
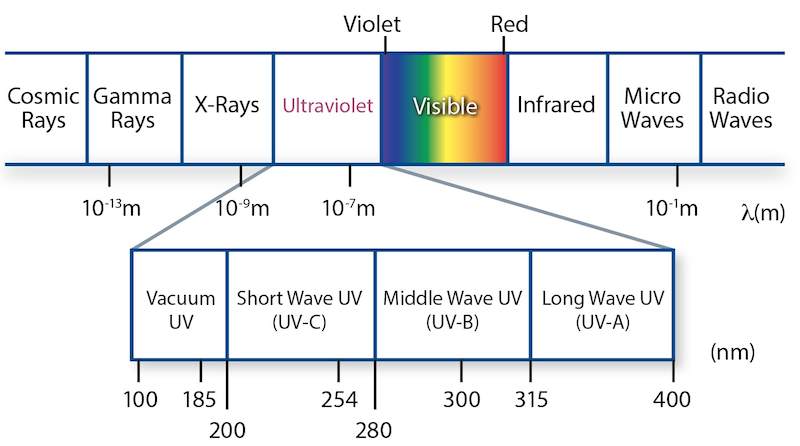
The wavelength range of the electromagnetic spectrum is called “ultraviolet radiation”, or UV radiation. It immediately joins to the diverting end of the visible range, the violet. The International Commission on Illumination (ICE) has defined the wavelength range between 400 nm (limit of the visible light) and 100 nm (the beginning of the area of the X-rays) as UV. The ICE divided the UV mainly under photo-biological aspects in three areas:
| Spectral | Wavelength | Photon Energy |
| UVA (near UV) | 400 – 315 nm | 3,15 – 3,94 eV |
| UVB (medium UV) | 315 – 280 nm | 3,94 – 4,43 eV |
| UVC (far UV) | 280 – 100 nm | 4,43 – 12,4 eV |
UV Applications
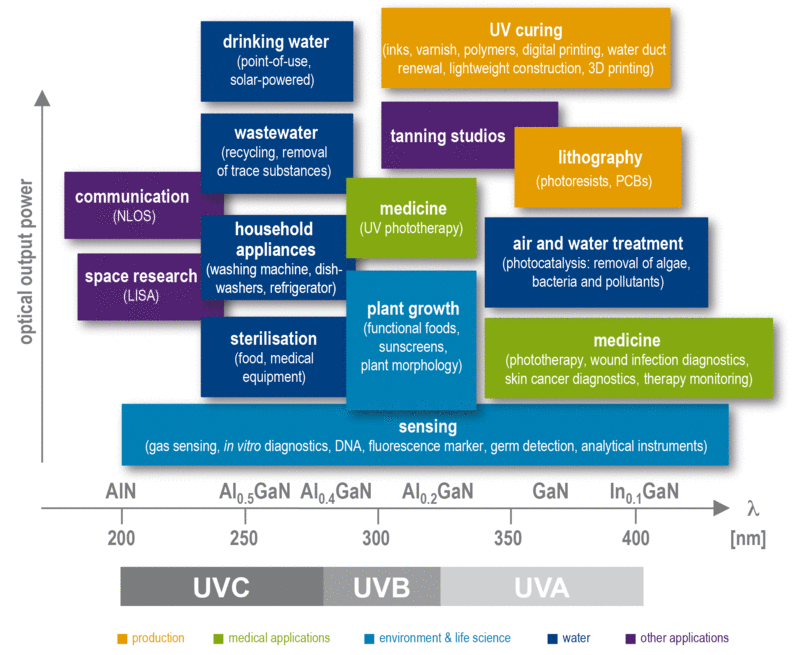
UV radiation is more energetic than visible light. Its energy is sufficient to destroy chemical bonds or to generate reactants and to enable them to enter into new bonds. Since chemical bonds are of different thickness, different energy or wavelength-specific binding can be selectively excited or broken, and thus different chemical or biological processes can be controlled by means of UV light.
Different application areas have specific requirements for UV components and UV systems, since not only the wavelength of the radiation is crucial, but also, depending on the application, certain minimum irradiance or irradiation (dose) must be achieved. Furthermore, areas of different size, including in relation to the radiation source very large areas, often have to be homogeneously illuminated (eg, UV-curing, water treatment, air purification, surface disinfection, etc.). In contrast, applications in sensor area require narrowband UV-ray point sources and wavelength-sensitive detectors.
In the control of chemical and biological processes by exposure to radiation the use of chemicals can be completely dispensed. UV irradiation affects neither smell, nor taste, color or pH of the irradiated material and leaves no material additions. Thus, the use of UV irradiation is particularly attractive for the purification of drinking water, producing sterile food and drugs, the provision of clean air as well as in the field of hygiene (eg in hospital).
Besides the use of UV radiation for chemical and biological synthesis or degradation processes, it can also be used for the analysis of solids, liquids and gases. Narrowband UV emitters are well suited for the selective quantitative determination of specific ingredients, such as polluting gases in the atmosphere or nitrogen oxides and sulfur dioxide. UV irradiation can cause fluorescence, which means the emission of radiation having a different wavelength than the incident. This is often characteristic of pathogenic germs, drugs or other hazardous materials and can be used for specifically identification and quantitative analysis of certain substances or products, for example, in safety engineering and the chemical and medical analysis. By focusing optical or expansion of the radiation field the spatially resolved excitation of selected regions is possible.
In the interaction with the biological tissue UV radiation, depending on the dose and the wavelength can lead to both beneficial as well as adverse effects. Wavelength and intensity of UV radiation sources can be optimized for any application in question (eg phototherapy), so that harmful side effects are ruled out or in comparison to currently available UV-sources significantly reduced. This opens up the field of medicine diverse applications in the diagnosis, therapy monitoring and patient monitoring.

 UVFAB Systems, Inc.
UVFAB Systems, Inc.